44+ how to calculate van't hoff factor for nacl
Here we will use ideal van t Hoff factors. Vant Hoff Factor for the.
What Is The Van T Hoff Factor For The Nacl In Aqueous Solution Assuming The Complete Dissociation Of Nacl Quora
Web The Vant Hoff factor can be defined as the ratio of the concentration of particles formed when a substance is dissolved to the concentration of the substance by mass.

. Determine the number of moles q of released ions. Web To be quantitative we introduce the vant hoff factor i. Web Step 1.
M is the molality of the solution. The dissociation of solute molecules into ions results in an. Web Vant Hoff explained that solute dissociates into ions when solutes are dissolved in a solvent.
Web moles of NaCl 2g NaCl 1 mol NaCl 5845g NaCl 00342 mol NaCl mass of water 98 g 0098 kg molality 00342 mol 0098 kg 0349 molkg Now we can. Revised equations to calculate the effect of ionization are then. Web One of the most common formulas used to calculate the value of the Vant Hoff factor is i moles of particles in solutionmoles solute dissolved.
Web Find step-by-step Chemistry solutions and your answer to the following textbook question. I actual number of particles in solution after dissociation number of formula units initially. Web Up to 6 cash back The vant Hoff factor is the number of particles that a single solute will dissociate into when added to a solution.
Calculate the molality and vant Hoff factor i for the following aqueous solutions. Web The actual van t Hoff factor is thus less than the ideal one. The sodium ion and the chlorine ion.
Calculate the molality of the. Web Finding the Vant Hoff factor from the formula of a chemical. Web The van t Hoff factor is the ratio between the actual concentration of particles produced when the substance is dissolved and the concentration of a substance as calculated from.
Analyzing the formula for salt we have index 1 in Fe and index 3 in Cl so the number of moles of ions. MgCl 2 will dissociate into three particles. Web Sodium chloride consists of two ions ie.
Web i is the vant Hoff factor Kf is the molal freezing point depression constant for the solvent and. So the Vant Hoff factor for NaCl considering complete dissociation is 2.

Supramolecular Polymers Historical Development Preparation Characterization And Functions Chemical Reviews

What Is The Van T Hoff Factor For The Nacl In Aqueous Solution Assuming The Complete Dissociation Of Nacl Quora
The Van T Hoff Factor
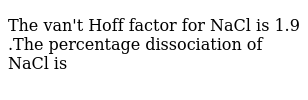
The Van T Hoff Factor For Nacl Is 1 9 The Percentage Dissociation Of Nacl Is

Aas Pdf Pdf Atomic Absorption Spectroscopy Atomic Orbital

Fast Capture Of Fluoride By Anion Exchange Zirconium Graphene Hybrid Adsorbent Langmuir
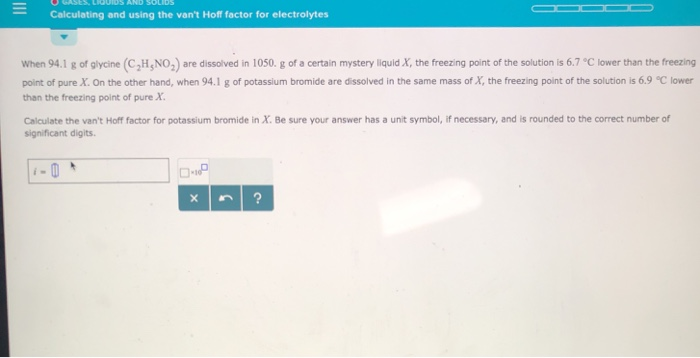
Solved Calculating And Using The Van T Hoff Factor For Chegg Com

Hydrogen De Absorption Improvement Of Nabh4 Catalyzed By Titanium Based Additives Request Pdf

Van T Hoff Factor I How To Calculate Van T Hoff Factor I Entrancei

Supramolecular Polymers Historical Development Preparation Characterization And Functions Chemical Reviews

How To Calculate The Van T Hoff Factor Of A Solute Quora

Calculate The Van T Hoff Factor When 0 1 Mol Nh 4 Cl Is Dissolved In 1 L Of Water The Deg Youtube

Calculate Van T Hoff S Factor For Chemistry Solutions 13995285 Meritnation Com

Van T Hoff Factor

Aleks Calculating And Using The Van T Hoff Factor For Electrolytes Youtube

Van T Hoff Factor I How To Calculate Van T Hoff Factor I Entrancei

How To Calculate The Van T Hoff Factor Of A Solute Quora